TUSCALOOSA, Ala. — Recent research from The University of Alabama sheds light on how a common industrial chemical and potent carcinogen begins the path to cancer.
The work led by Dr. John B. Vincent and Dr. Stephen A. Woski, UA professors of chemistry and biochemistry, could lead to methods to prevent the development of cancer from exposure to a variant of chromium, known as chromium(VI) or hexavalent chromium.
Chromium(VI) is an additive to steel and a coating in a large number of materials, and people can be exposed to it in industrial and construction settings or in contamination of drinking water. When inhaled, it is a potent carcinogen that can kick start cell mutation that results in cancer.
Despite years of study, the way chromium(VI) works as a cancer-causing agent is unclear, partly because the many ways it can damage DNA. One manner that has not been studied nearly as much involves the conversion of chromium(VI) to chromium(III).
“While chromium(VI) is a known carcinogen, how at a molecular level it initiates the start of the process leading to cancer is not known,” Vincent said. “This work is the first step of testing the potential toxicity and carcinogenicity of this manner of behaviors in cells.”
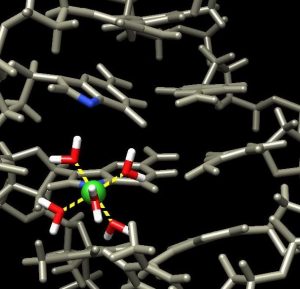
In a paper in the journal ChemBioChem, Vincent and co-authors show one way chromium(III) binds to DNA and how the techniques involved can be used to investigate other ways it binds.
Chromium(III), or trivalent chromium, has debatable nutritional value when ingested, but it is not toxic since it is flushed out of the gastrointestinal tract. However, chromium(VI) bypasses these natural elimination pathways and reduces to chromium(III), possibly causing the harm.
The next steps are to find other specific ways chromium(III) binds and potentially damages DNA.
“Once we characterize all these ways, then we can start to perform studies to see if each results in damage to DNA that can lead to cancer,” Vincent said. “If these chromium(III)-DNA complexes are found to be the primary cause of chromium(VI)-related cancer, then understanding them could lead to methods to prevent cancer from developing from them.”
The lead author on the paper is Dr. Silas Brown, who graduated with a doctorate from UA in August. Other authors on the paper include Dr. Michael K. Bowman, UA professor of chemistry and biochemistry, Dr. Molly M. Lockart, who also graduated with a doctorate from UA in August; and C. Sumner Thomas, an undergraduate research assistant.
Contact
Adam Jones, UA communications, 205-348-4328, adam.jones@ua.edu
Source
Dr. John B. Vincent, jvincent@ua.edu
